Effect of Microplastics on the Germination and Growth of Terrestrial Plants
Article information
Abstract
Objectives
The human beings of our globe are heavily reliant on the use of plastic products on a daily basis. Microplastics (MPs) are tiny particles that are formed as a result of plastic degradation. MPs can be found in terrestrial environment, but previous studies focused on the effects of MPs on marine environment and there are less studies on the effects of MPs on terrestrial environment. In particular, there is still a relatively limited number of studies on the effects of MPs on terrestrial plants. Therefore, this review paper aims to examine previous studies on the effect of MPs on the germination and growth of terrestrial plants.
Methods
This review summarized the previous findings on the effect of MPs on the germination and growth of terrestrial plants in order to identify the research gaps and trends on the effects of MPs on terrestrial plants.
Results and Discussion
Previous studies on the effect of MPs on the germination and growth of terrestrial plants were reviewed and the observations were summarized to find the gaps in this field of research. Previous studies showed that MPs can change soil properties and these changes can be different depending on the shape and size of MPs. MPs can also release chemicals such as phthalates and heavy metals that might have toxic effects on terrestrial plants. Different forms, types, and sizes of MPs were used in previous studies to study the effects on seed germination and growth of different plants. Most studies reported that MPs have negative effects on seed germination and plant growth (e.g., biomass, chlorophyll contents). Smaller MPs tend to have more significant effects than larger MPs. The changes in the soil properties due to MPs can change microbial community structures and this, in turn, may affect the soil-plant interaction.
Conclusion
This study summarizes what have been reported in the previous studies on the effect of MPs on seed germination and growth of terrestrial plants. MPs are likely to affect seed germination and plant growth negatively, and this will eventually lead to reduction in food production and food quality. Owing to the various types, forms, and sizes of MPs that could be present in the terrestrial environment as well as the chemicals that could be released from MPs, there is much to explore to better understand the effect of MPs on terrestrial plants and the underlying mechanisms of such effects.
1. Introduction
The human beings of our globe are heavily reliant on the use of plastic products on a daily basis. Plastic production is predicted to increase in the future to keep up with the world's rising living standards [1]. However, majority of these plastics(~85%) are not recycled and end up in the terrestrial environment with 20-42% of the whole global plastics stored within the land [2-4].
Microplastics (MPs) are tiny particles that are formed as a result of plastic degradation [5]. Different institutes have different plastic classification systems, which are based on the dimensions of MPs (Table 1). In general, plastics less than 5 mm are considered as microplastics [6,7]. Different types of microplastics such as polyethylene (PE), polyvinylchloride (PVC), polystyrene (PS), polypropylene (PP), and polyethylene terephthalate (PET) are found in the environment [8].
MPs are found in various terrestrial environments including agricultural fields, and these MPs come from different sources including sewage sludge, plastic mulch, street runoff, litters, landfill leachate, personal care products containing microbeads, paint, and atmospheric dust [9]. Improper use and handling of wastes can also directly introduce MPs into soil [9]. Many studies also found that the number of MPs detected in sludge was higher than those found in raw and treated wastewaters [9]. This implies that the use of sludge as fertilizers can introduce MPs into terrestrial environment.
Recent studies reported that the effects of MPs on marine environment have been extensively studied but there are less studies on the effects of MPs on terrestrial environment [10], thus, there needs to be more studies on the effect of MPs on terrestrial environment [11]. The impacts of MPs on marine organisms and terrestrial organisms can be different. Many studies reported adverse effects on different marine organisms including fish [12]. Fish, in particular, have gills where MPs in water are filtered and accumulated, which will block the gills causing adverse effects on fish [12]. Also, there are more natural weathering processes that can contribute to the production of MPs and nanoplastics in the marine environment [13]. For example, floating plastics can be chemically degraded by sun light and physically degraded by waves [13]. Thus, MPs can be more easily generated in the marine environment than in the terrestrial environment. Although studied less than marine organisms, the impacts of MPs on terrestrial organisms such as plants, earthworms, and bacteria have been studied [14]. Among different terrestrial organisms, the effects of MPs on terrestrial plants have been investigated extensively [14]. However, when compared to the marine environment, there is still a relatively limited number of studies on the effects of MPs on terrestrial plants. Therefore, this review paper aims to examine previous studies on the effect of MPs on the germination and growth of terrestrial plants to find the gaps in the current studies and suggest the future research needs.
2. Effect of MPs on soil environment
2.1. Effect of MPs on soil properties
The presence of MPs in soil environment can change soil properties largely due to the dimensions and shapes of MPs [15]. The major form of MPs found in the soil environment and sludge was fibers (i.e., 97% in soil, 90% in sludge) [15]. So, fibers can be found in soil amended with sewage sludge [16]. In fact, MPs concentrations of 30.7 × 103 particles (kg dry sludge)-1 has been reported [17]. A large amount of plastic mulch is used in agricultural fields, so the concentrations of MPs in soil may also increase [18].
The size of MPs is one of the important factors that contribute to the effects of MPs. The exposure to larger MPs (e.g., 5 μm) significantly reduced the root biomass of broad beans without genotoxicity, but smaller MPs (e.g., 0.1 μm) exhibited significant genotoxicity, although the root biomass was unaffected [19].
The changes in soil properties such as particle density, bulk density, and pH due to the presence of MPs may have a significant effect on soil organisms and the biophysical parameters of the soil. MPs are incorporated into the soil through soil biota, and this will affect plant development and soil biological activities [20]. Some previous studies reported increases in soil aggregation (i.e., by ~18%), soil pH (i.e., by ~4%), and nutrient retention (i.e., by up to ~70%) due to the presence of MPs [21]. Another study reported that the presence of 0.48 μm PE MPs decreased soil pH and water holding capacity [22]. Also, some negative impacts on soil enzymes, seed germination, and plant growth were reported [22].
There are a few review articles on MPs in the terrestrial environment, and these review articles focus mainly on the origins, occurrences, fates, potential ecological and economic implications, and future prospects of MPs in terrestrial environment [22,23]. Therefore, this review article is set to gather and analyze the reported effects of MPs on terrestrial plants, so that this review can identify the gaps in current study and make suggestions for future research on the effects of MPs on terrestrial plants.
2.2. MPs as a chemical carrier
MPs can act as a chemical carrier in the environment: MPs can either release some chemicals (e.g., additives) from MPs or adsorb chemicals on the surfaces of MPs [24]. The plastic ingredients such as additives may leach out during the disintegration of plastics, contaminating soils and waters [24,25]. For example, phthalates such as (di-2-ethylhexyl) phthalate (1.04 mg kg-1) and diisobutyl phthalate (0.16 mg kg-1)) could be released into greenhouse soils [26]. Also, bisphenol A (BPA), a harmful endocrine toxin, can be released from MPs to soils [26]. The presence of MPs in soils could also affect transport patterns of chemicals in soil [27]. MPs can adsorb chemicals on their surfaces reducing the mobility of chemicals in soil [28]. For example, the bioavailabilities of Cu, Cr, and Ni were reduced when MPs were present in soil [28]. On the other hand, MPs increased the mobility of organic pollutants (e.g., polychlorinated biphenyls (PCBs)) in natural soil columns [29]. MPs can change soil characteristics as well. For example, the introduction of PE MPs (i.e., 28% w/w) significantly increased the levels of dissolved organic carbon, nitrogen, and phosphorus in soil [30]. Furthermore, the phytotoxic chemicals in MPs can cause a hydrophobic barrier in soil, resulting in harmful effects on plant roots and soil microbes [31]. The mineralization rates of soil can be inhibited by MPs [32]. Some researchers have also observed metal migration from MPs, although such studies are relatively limited compared to the studies of organics. A recently published research has shown that several kinds of metals (Cr, Cu, As, Pb, Ba, and Sn) migrated from plastic film fragments and that the presence of prothioconazole (a fungicide) promoted the release of metals [32]. The release rates of metals increased dramatically when the particle size decreased [33].
3. Effect of MPs on terrestrial plants
3.1. Effect of MPs on seed germination
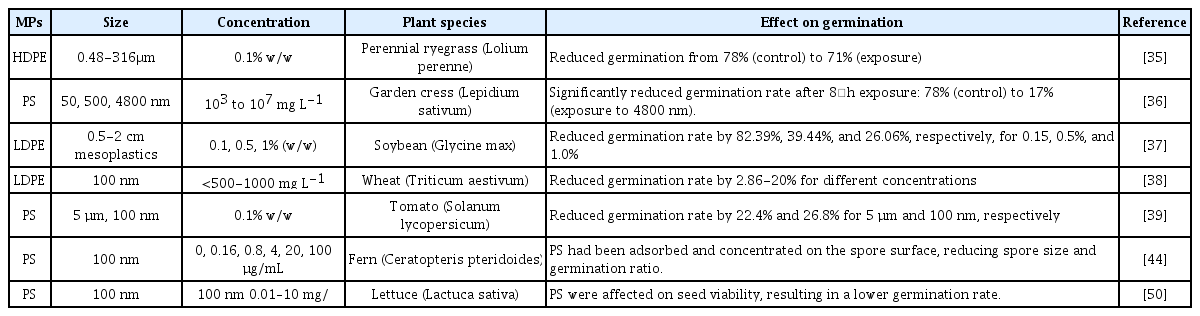
The seed germination can be affected by the exposure to MPs [34]. For example, the seed germination of wheat and perennial ryegrass was reduced after exposure to MPs [35]. The germination rate of garden cress was reduced from 78% (i.e., control) to 17% after exposure to 4800 nm PS MPs [36]. Furthermore, the viability of the seed germination decreased with increasing plastic concentrations in soil [37]. For examples, the soybean seed germination viability decreased to 82%, 39%, and 26% when exposed to 0.1%, 0.5%, and 1% PE in soil, respectively [37]. However, some studies observed beneficial effects of MPs on germination [38]. Compared to the controls, the germination of wheat seeds was inhibited by 2.9-20% when exposed to MPs at <500 mg L-1, while higher MPs concentrations (1000 mg L-1) promoted seed germination [38]. The reduction in the germination rates after exposure to MPs may be explained by the physical blockage of the pores [39].
Different types MPs showed different toxic effects on plants. For example, the seed germination of perennial ryegrass was 45%, 74%, and 75% after exposure to HDPE, clothing fiber, and biodegradable polylactic acid (PLA) MPs, respectively [40]. Furthermore, the effect of the mixture of different types of MPs is not necessarily greater than that of the individual MPs. For example, the germination rate of garden cress plants was by 55% and 55.3% when exposed to PE and the mixture of PE and PVC, respectively, and this may suggest a greater toxic effect of PE than PVC [41]. Table 2 summarizes some of the previous studies on the effect of MPs on germination.
3.2. Effect of types of MPs on plant growth
The changes in the soil properties such as moisture content, density, and structure due to the introduction of MPs to soil can affect root characteristics, growth, and nutrient uptake by plants [42]. The effects of MPs on plants can be different by the types and concentrations of MPs. As shown in Table 2, MPs tend to have negative effects on the initial phase of plant growth, and MPs can further affect the growth of plants. Previous studies showed both positive and negative effects on the growth of roots and shoots (Table 3). In one study, MPs of 100 nm positively affected the biomass of wheat roots and shoots [43]. Also, when onions were exposed to PS MPs, the root length was increased, but the average root diameter was reduced [44]. On the other hand, the exposure to various types of MPs resulted in the reduction of the rice stem biomass [45], root hair length and density of white clover [46], biomass of cucumbers [47], and of root and shoot biomasses of beans [47]. Similar results were also observed with other plants such as maize and lettuce [46,47]. The exposure of plants to MPs can also have effects on the chlorophyll contents of plants [48,49]. For example, the chlorophyll contents and soluble sugar of cucumber were significantly decreased after exposure to 100 nm PS [49], and the leaf chlorophyll content of maize was decreased after exposure to HDPE [50].
3.3. Effect of size of MPs on plant
The toxic effect of MPs on plants largely depends on the sizes of MPs. Generally, the toxic effect of MPs on plants increases with decreasing MPs size [50]. For example, in comparison to 100 nm PS, 5 μm PS had lower negative effects on tomato germination [51]. Also, the inhibition of the root and shoot elongation of wheat was greater by 100 nm PS than 5 μm PS [51]. The 100 nm PS resulted in the greater inhibition of the shoot and root biomasses of cucumber than the 300, 500, and 700 nm PS [52]. The green fern spore size and germination ratio were decreased by PS nanoplastics with a size of 100 nm [53]. Broad bean roots showed lower growth and genotoxic impairment after exposure to PS nanoplastics with a size of 100 nm [54]. When exposed to LDPE MPs with a size between 30~40 µm, duckweed plants showed mechanical blocking of roots and lower the root growth [45]. When exposed to 0.5~6 µm PS MPs at 250 mg mL-1, the green algae growth was reduced by 45% [45]. Previous studies showed that, compared to MPs, the effect of nanoparticles (NPs) on plant (e.g., roots size, stem size, total biomass of shoot and roots) can be more significant [38].
3.4. Effect of MPs on the relationship between soil microorganisms and plants
Plant performance relies strongly on soil microorganisms and their diversity [45]. MPs and microfibers can have impacts on the processes of soil formation, stabilization, and disintegration of soil aggregates, and this, in turn, is expected to have implications for microbial development [45]. Plant can rely on root-colonizing bacteria, such as nitrogen fixers, pathogens, and mycorrhizal fungi [55]. If MPs cause changes in soil structure, this could have an impact on soil microbial community, which would undoubtedly affect soil functions such as mineralization rates and root-colonizing symbiont groups [55]. Furthermore, MPs can have a direct toxic effect on arbuscular mycorrhizal (AM) fungi). Adding low-density PE MPs in soil significantly affected the composition and abundance of microarthropod and nematode communities [55]. In addition, different forms of MPs can have different effects on soil microbial structure [55]. For instance, the application of PE powder (150-250 μm, 7%(w/w)) did not change bacterial community structure significantly; however, the inclusion of PE microfibers (<2 mm) led to an increase in soil microbial diversity [55].
4. Future perspectives
Further research is needed to find out how plants respond to MPs in order to address the knowledge gaps related to MPs and plants. Previous studies on the effect of MPs on terrestrial plants focused on a single plant species, such as perennial ryegrass, common wheat, lettuce, broad bean, carrot, and spring onion. Also, these studies used a variety of experimental conditions including varying soil textures and MPs characteristics such as concentrations. Thus, the findings are limited to certain types of plants and certain characteristics of MPs. Since there is currently no established protocol for identifying and measuring soil MPs, it is challenging to compare the previous studies to each other. Therefore, further studies are needed on the effects of MPs on more species of plants, the effects of interaction between MPs and other chemicals on plants, and on the fate of MPs on terrestrial environment. Additionally, the accumulation of MPs in plants may threaten terrestrial organisms through trophic transfer. Future study should, therefore, include the impacts of MPs on food security. Furthermore, it is necessary to establish standard procedures for extracting and quantifying MPs of different properties (e.g., size, polymer types, shapes) in terrestrial environment.
5. Conclusions
MPs are considered as a global environmental contaminant, yet there is limited understanding of the effect of MPs on terrestrial ecosystems. Previous studies suggest that MPs can have various effects on terrestrial plants and these effects can be the results of the changes in soil properties due to MPs. MPs are likely to affect seed germination and plant growth negatively, and this will lead to reduction in food production and food quality. Owing to the various types, forms, and sizes of MPs that could be present in the terrestrial environment as well as the chemicals that could be released from MPs, there is much to explore to better understand the effect of MPs on terrestrial plants and the underlying mechanisms of such effects. Also, once MPs are absorbed by terrestrial plants, they can be taken by animals along the food chains, and this might eventually reach human imposing some risks. However, there is very limited studies on this. Therefore, the effects of MPs, individually and in mixtures, on terrestrial plants, need to be explored further to determine the risks on terrestrial plants and proper management strategies need to be prepared using these data in future. In doing this, standardized methods, which are not yet available, need to be employed as well.
Acknowledgements
This study was funded by the National Research Foundation of Korea (NRF-2021R1A2C4001746).
Notes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.