1. Introduction
Eichhornia crassipes, commonly known as water hyacinth, is an aquatic floating macrophyte, native to the Amazon basin. The plant is hardy and very difficult to eradicate because it can survive extremely harsh conditions. It can absorb into its tissues large quantities of minerals from the water column. Plant and plant derived products form an important alternative medicine category in health care that are often used for treatment of several disorders. According to folk medicine, Eichhornia crassipes has been used for treatment of several aliments. In Java, green parts, inflorescences and flowers spikes are cooked and eaten. There are reports of its use as a styptic in Chhattisgarh, India. The fresh juice of this weed is used to treat fresh wounds as the tribes believe that it stops further spread of infection and relieves stress associated with the injury. Along with vinegar, it has been reported to use in treatment of septic wounds [1]. Eichhornia crassipes has also been used to ease swelling, burning, haemorrhage, and goiters. In India and Nigeria, the oil from flowers has been used for skin care as well as for treatment of skin diseases [2]. In Assam, the flowers and roots have been used in treatment of stomach ache and pneumonia. In Bangladesh, the plant has been used in treating hepatic disorders [3]. In recent years, there are reports of Eichhornia crassipes being used as an insecticide, antioxidant, antitumor, antiinflamoatory and as an antimicrobial agent [4-6].
Trace elements constitute less than 0.01% of the weight of the human body; however, despite their relative scarcity, they play an important role in human growth and development deficiency or excess of trace elements are associated with several metabolic disorders. An investigation focusing to understand the relationship between trace elements and human health has led to the conquest of diseases such as pernicious anaemia and recently, Keshan disease.
The purpose of this study is to determine the distribution of trace elements in different parts of the Eichhornia crassipes. It is expected that the metal distribution within the plant species would be a representation of the concentration of the water in which it is found. Majority of available literature on water hyacinth is focused on its potential in phytoremediation, as a source of cellulose for paper production or as a source of cattle feed/fish feed. The plant has been exploited only to a certain extent in terms of its phytochemical uses [7]. A more systematic investigation needs to undertaken to exploit the ability of Eichhornia crassipes to accumulate non-essential and essential trace elements in order to investigate its potential for specific pharmaceutical or cosmeceutical studies like diarrhoea, skin burns, antibacterial agent, goiter etc [8-11]. Although, only elemental analysis data is insufficient to decode the mechanisms involved in the use of Eichhornia crassipes in folk medicine, and a more detailed study needs to be undertaken with respect to its secondary metabolite profiling. Nevertheless, insight into the trace element concentration of this plant can perhaps be harnessed to postulate the rationale behind its use in traditional medicine. The occurance of high levels of trace elements in Eichhornia crassipes could perhaps motivate government or private sectors to invest in the removal of the weed and use it for extraction of the trace elements for commercial purpose. This is the first study reporting concentration levels of trace elements in Eichhornia crassipes, particularly from India.
2. Materials and Methods
2.1. Collection of plant material
Eicchornia crassipes was collected in the month on March 2018 from three different locations across Pune district, viz, Pashan Lake, Mula river, Mutha river, Pune, India. The samples were authenticated by department of pharmacognosy, Poona College of Pharmacy, India. The leaves, petioles and roots of the plant were segregated and set to dry under shade for 15 days until constant weight of the dry mass was obtained. The dried samples were then ground to fine powder.
2.2. Sample preparation and digestion
The samples were separately weighed, following which they were subjected to microwave digestion (Mars Express Vessel) according to the parameters given in Table 1.
For digestion, 0.5 g of sample was taken into a digestion vessel to which 3 mL of nitric acid (HNO3 69% Suprapure grade), 7 mL of hydrochloric acid (HCL 30% Suprapure grade) was added. Blank solutions were prepared similarly. Digested samples were transferred to falcon tubes and diluted with acidified water to a volume of 50 mL. The resulting solutions were subjected to Inductively Coupled Optical Emission Spectroscopy (ICP-OES) and Inductively coupled Plasma Mass Spectrometry (ICP-MS) analysis.
2.3. Iodine sample preparation
For Iodine determination, 0.5 g of the sample was digested in basic medium by taking 1 mL tetra methyl ammonium hydroxide (TMAH) to which 5 mL of water was added. The solution was subjected to centrifugation, after which it was kept in oven for 2 h at 80℃. The final volume was made up to 50 mL water.
2.4. Standard preparation
Stock solutions containing 1,000 mg/L of Perkin Elmer Pure was used to prepare different custom-grade multielement standard solutions for Co, I, Se, Ni, Cu, Mn, Zn, Fe by diluting with 50 mL acidified water. Calibration curves for each element were constructed in triplicate using six different concentration. An internal-standard stock solution containing 100 µg/L of rhodium (Rh) was prepared from single-element stock solutions. NIST Standard reference material SRM 1547 (Peach leaves) was used for establishing accuracy of the analytical method. Suprapure grade nitric acid and hydrochloric acid were used for preparing stock solutions of 100 µg/mL and 10 µg/mL for ICP-OES and ICP-MS respectively.
2.5. ICP-OES and ICP-MS analysis
Initially, the sample digests were subjected to a semi-quantitative analysis which allowed the establishment of the range of concentrations of the elements in the various digestion solutions. The error associated with the semiquantitative analysis was calculated to approximately 25%. Based on the results obtained, the elements were divided into two groups depending on the determined concentrations. Group 1 elements (Co, Ni, Cu, I) were determined by ICP-MS (Agilent 7700 series) and group 2 elements (Mn, Zn, Fe) were subjected to ICP-OES (Thermo Fisher Scientific, Bremen Germany, i CAP 6300) analysis. The quantitative determinations were carried out by a calibration curve using multielement standard solutions in which the concentrations of the elements were in an optimal measurement range.
3. Result and Discussion
3.1. Choice of isotopes and correction of interferences
After careful evaluation of all possible isotopes, their abundances, potential interferences (meaningful in the analysis of plants) and the corrections introduced, isotopes selected for quantitative estimation of the respective elements were; 59Co, 127I, 60Ni, 66Cu. Pure single element standard solutions of the interfering elements were used to establish the actual interferences on all isotopes under investigation.
Where Scor is the corrected signal of the analyte; Smeas is the measured signal; Sinter is the signal of the interfering element and A is the % of formation of the respective interfering species determined in the solution of the interfering element at the working conditions specified in Table 2 and Table 3. Sinter is measured in each sample and the isotope abundance is accounted for.
3.2. Element concentration
The concentration values of Co, I, Se, Ni, Cu, Mn, Zn, Fe in various parts like roots, leaves and petiole as expressed in mg/Kg are given in Table 4. The total range of concentration of elements was found as low as 0.94 mg/Kg for I and as high as 566 mg/Kg for Mn. The order of overall mean concentration of trace elements in Eichhornia crassipes was found as Mn > Fe > Zn > Cu > Ni > Co > I. Roots of the plant are in direct contact with the surrounding water and as a result they form the principle part of element uptake. Concentration of all measured elements was found to highest in roots. The concentration of Ni, Cu, Mn, was found to be highest in Roots > Petiol > Leaves. Whereas, the concentration of Zn and Fe was found to be highest in roots followed by leaves and petiole. This finding suggests alteration in transport mechanism or storage process involved with Zn and Fe. More detailed studies involving 2D-element mapping are necessary to evaluate the transport and storage mechanism of these elements in Eichhornia crassipes. Concentration of Co was high in roots but in petiole and leaves it was almost similar. An interesting finding was the occurrence of measurable quantities of Iodine in all the parts of the plant despite its non marine source. Occurrence of measureable Iodine in ecosystems far from seashores is not a rare phenomenon. Although high levels of iodine invariably occur in vicinity of costal zones, nevertheless, there is no simple correlation between distance from shoreline and content of iodine in a sample. Among many factors that influence the level of iodine in a sample, supply of iodine, sample’s iodine fixation potential and adsorption of atmospheric iodine on plant’s surface are some important contributors [13]. This data shows that amongst all measured elements, Eichhornia crassipes is exhibited highest bioaccumulation for Mn. This finding is in agreement with another similar study done by Olutona et al. [14].
3.3. Recovery
Recovery value of an analytical method, is a parameter that signifies accuracy of the adopted analytical process. Accuracy is defined as the closeness between the true value of the analyte in the sample and the experimentally obtained value by following the prescribed analytical procedure. A good recovery value ensures that no significant loss or contamination has occurred during the test procedure.
To establish accuracy of the method, certified reference standard SRM 1547 (Peach leaves) was measured and concentration of the desired elements were determined using both ICP-MS and ICP-OES. Good agreement between certified values and measured values was observed (Table 6). This demonstrates the appropriateness of the proposed method for applicability in routine element determination in plant samples.
Recovery values for the desired elements were also calculated in the digested plant samples of leaves, roots and petiole after spiking with 100% of the target limit by standard element solution.
The recovery values obtained for all the measured elements in different parts of Eichhornia crassipes, are shown in Table 7. Recovery values obtained were in between 95% to 101%.
3.4. Limits of detection and quantification
The calibration curves for all the analytes were built on six different concentrations and LOD and LOQ values were determined.
The limits of detection (LOD) were calculated by taking three times the standard deviation of the 10 individually prepared blank solutions.
3.5. Linearity
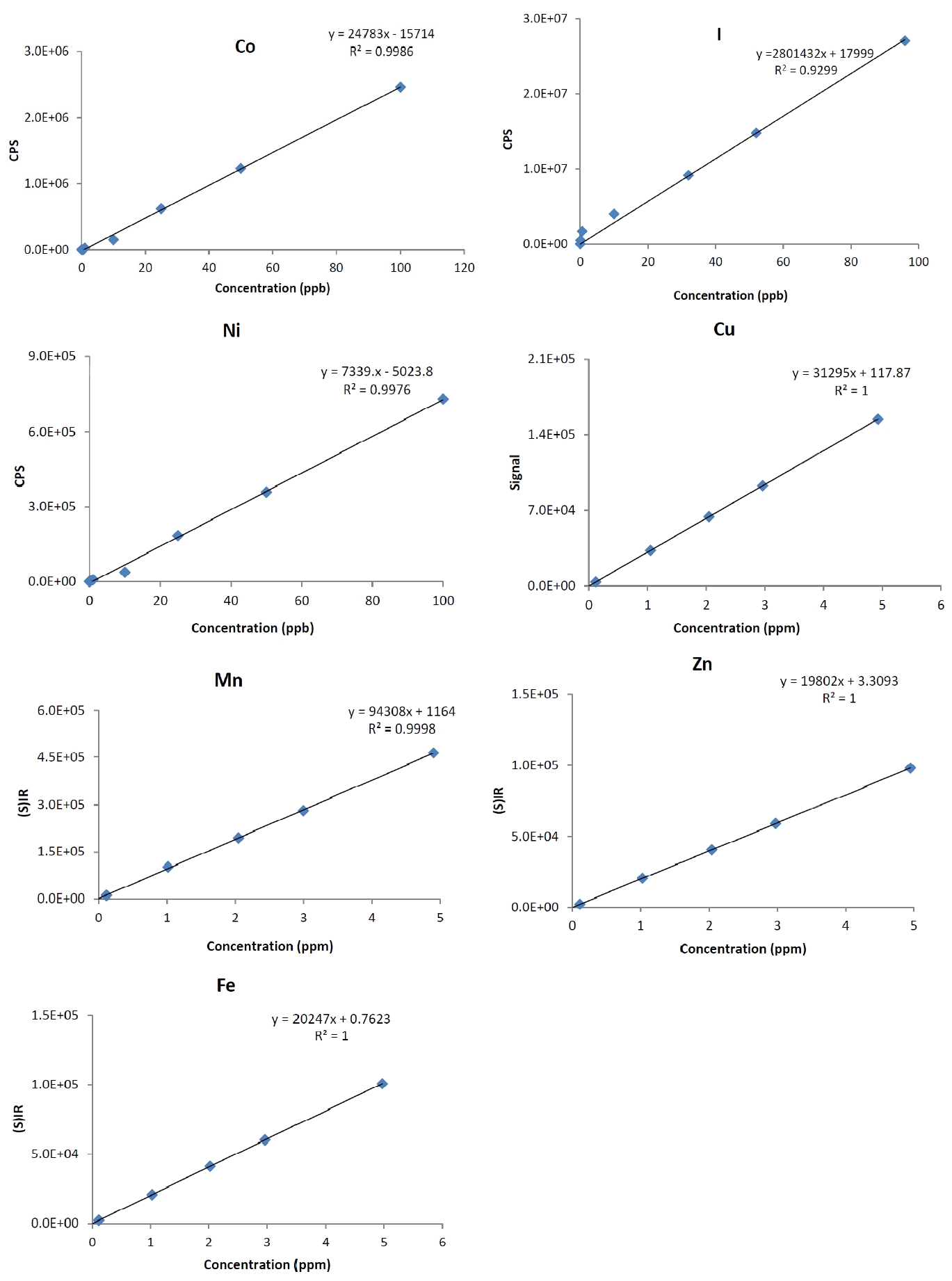
The correlation coefficients were calculated for the calibration curves for all the seven trace elements on six standard replicates for six different concentrations (Fig. 1). Concentration used for determining linearity were between 10% to 120% of the target limit for each element in the final analysis solution. R2 values for the individual elements are shown in Table 5. Good correlation was obtained for all the measured elements. Observed R2 values were in the range of 0.9976 to 1.
4. Conclusion
Eichhornia crassipes has been long regarded as an invasive weed. Majority of available literature on water hyacinth is focused on its potential in phytoremediation, as a source of cellulose for paper production or as a source of cattle feed/fish feed. The plant has been exploited only to a certain extent in terms of its phytochemical uses. We believe our understanding of this plant is still relatively weak, which is limiting its appropriate management. By performing this study, we have attempted to begin to decode the use of this plant in folk medicine by correlating it use to the occurrence and content of trace elements in various parts. Minor and trace element analysis of the plant will provide evidence based rationale behind the use of Eichhornia crassipes in treatment of various aliments like goiter, antimicrobial, anti-inflammatory, antioxidant, which will present the basis for its potential use in modern treatment.
In this study a validated ICP-OES and ICP-MS method was established for determining the minor and trace element concentrations of Co, I, Ni, Cu, Mn, Zn, Fe in roots, petiole and leaves of the plant. Levels of all elements were found to be higher in roots, followed by petiole and leaves. Except for Zn and Fe, where concentration in leaves was found to be higher than in petiole. Of all the measured elements, total conentration of Mn was found to be highest (566 mg kg-1), followed by Fe (341 mg kg-1), Zn (40.26 mg kg-1), Cu (28.04 mg kg-1), Ni (9.54 mg kg-1), Co (4.33 mg kg-1) and I (0.94 mg kg-1).
Presence of iodine in the plant although surprising, provides rational for the use of this plant in treatment of goiter. High concentration of Mn, Cu, in the plant explanis could explain its use as antioxidant, antibacterial agent respectively.
Eichhornia crassipes can be thus be considered as potential source of nutrition and mineral supplements. However, the mineral composition of the plant obtained here was based on chemical analysis only. Biological evaluation using human and animal feeding studies would be required to establish the nutritional value of this plant as a source of minerals. Although we are fully aware that it has been widely acknowledged that this plant accumulates heavy metals from contaminated water sources, if the utility of the plant in pharmaceuticals/cosmeceuticals is established, it would be worthwhile to cultivate this rapidly reproducing plant in neutral water bodies for this purpose. A more detailed scientific protocol for evaluation of Eichhornia crassipes with respect to its phytochemical composition and pharmacological/cosmeceutical activity evaluation along with secondary metabolite profiling, is under process by the authors. With more such studies, a plant that has been considered a threat to the environment and economy, could be constructively harvested.